Simplify Regulatory Strategy & Change Management
Get up-to-date Requirements. Save time. Control risks.
What we do
Vistaar provides Global Regulatory, Clinical and Compliance Requirements to Pharma/Biotech, Devices, Diagnostics, OTC, Consumer Health, Cosmetics and Digital Health. With a solid combination of smart technology and subject experts, you will get up-to-date requirements and trusted insights accurately and much faster. Over many years, Vistaar has built deep data sets to help you do the right strategy. All this is done at the best price point in the industry.

Countries Requirements

Regulations & Guidance Documents
Clinical Trials

Marketed Product Details

Database
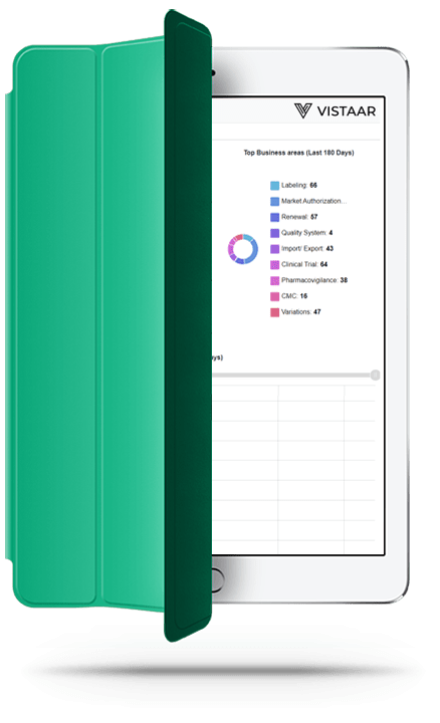
How Vistaar works

Technology
Vistaar automates heavy manual processes using Automation & Artificial Intelligence (AI) to help you manage regulatory change and compliance proactively and ensure timely mitigation in your organization. End-to-end Digital design automates and simplifies data acquisition, transformation, reporting and sharing .

Global Resources
Our global team of Analysts and Subject Experts, with expertise across a diverse range of industries, geographies and policy areas, collaborate with functional specialists to address questions about regulations including purpose, applicability, requirements highlights and more. All reports are vetted to ensure data and summaries are accurate and up-to-date.

Value to you
-
Improve Regulatory Foresight
-
Cut down Alert Noise
-
Unifying UI for full coverage of Regulatory value chain
-
Data & Workflow Automations to reduce manual work
-
Lower Total Cost of Ownership (TCO)
Benefits
- Get digital centralized library that is comprehensive and covers all applicable regulations you need to be compliant with today and going forward
- Stay on top of important regulatory developments that have direct impact on your products and organization
- Increase accuracy and timeliness of your regulatory and compliance updates
- Gain operational visibility and optimize processes
- Create a traceable matrix of laws, regulations, and guidance to do better impact assessment when changes come in
- Reduce the noise with smart, configurable alerts that target only those regulatory changes that affect you
- Spend up to 70% less time on process reviews by automating time-consuming tasks
- Avoid being caught off-guard by frequent changes that could affect your products (both in R&D as well as market) and compliance programs
- Know exactly what incoming regulatory changes apply to your organization and review internal Policies / Processes accordingly
- Assign to teams and track the status of each important change/alert
- Convert to documents (to share to other members) or projects (if the change is presumed big)
- Link Regulatory processes and Intel to Business Objectives
- Increase team productivity by having one platform for a more collaborative approach to compliance and risk management across the company (and subsidiaries)
- Understand and properly communicate the regulatory risks associated with your organization’s objectives and how to mitigate those to attain business goals.
- Effectively manage all these within budgets allotted
From the Blog
Pharma/Biotech – USA, …

Consumer Health – USA/…